MetaSystems at GfH 2025
MetaSystems Japan
Successful Installation at Laboratorios Carpermor
Impressions of the TGA 2024
Greetings from Nijmegen, NL
Middle East Meeting 2024
MetaSystems Sustainability Report 2023
Did you know that in 2019, the combined Scope 1 and 2 greenhouse gas emissions from MetaSystems and MetaSystems Probes were equivalent to only 4.1 average German individuals? Moreover, this emissions figure dropped to a mere 2.6 people equivalent in 2021. And in 2022, all the electricity consumed by MetaSystems and MetaSystems Probes was sourced from renewable energy reserves or was self-produced.

1,000th SlideFeeder x80
Technical Training at Headquarters
Greetings from the U.S. Applications and Support Team
Training in Buenos Aires
Two-day Hands-on FISH Workshop
Hands-on FISH Workshop
Outlook for 2023
The MetaSystems Year 2022
MetaSystems Distributor Meeting 2022
Collaboration with MLL on AI-based Karyotyping
Deliveries to Russia
Log4j Security Gap
'Phishing' Attack on MetaSystems Accounts
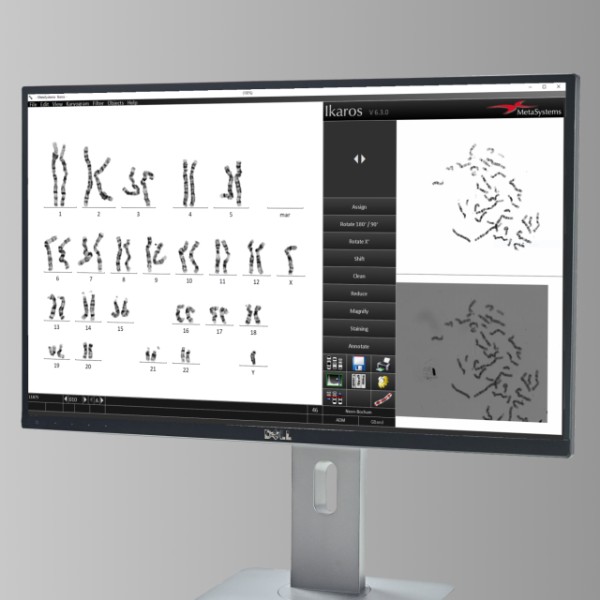
MetaSystems Announces Grant of US Patent for AI based Chromosome Analysis
Artificial intelligence opens entirely new horizons for image analysis. In the latest versions of Ikaros, MetaSystems has implemented Deep Neural Network (DNN) based artificial intelligence that greatly facilitates the processing of metaphase images for karyotyping. MetaSystems is proud to announce that our innovative DNN-based technology has now received a patent entitled ‘Methods for Automated Chromosome Analysis’” (U.S. patent no. 10,991,098).