Scoring Micronuclei
Automated Micronucleus Tests
Metafer-based analysis of in-vitro and in-vivo micronucleus samples for biodosimetry, toxicology, and mutagenicity research.
Micronucleus (MN) tests are used in toxicological screening and radiation biodosimetry to quantify the DNA damaging capability of an agent. The assays are now recognized as the most successful and reliable tests for genotoxic carcinogens. There are two major models of MN tests, namely (a) the cytokinesis-block MN assay (further referred to as the in-vitro MN assay), and (b) the rodent erythrocyte MN assay (further referred to as the in-vivo MN assay).
The Metafer software manages smart workflows that can do complete, unattended, and reliable image acquisition and analysis for both tests. Automated MN analysis is compliant to the respective OECD guidelines, and it can be performed in accordance with Good Laboratory Practices (GLP).
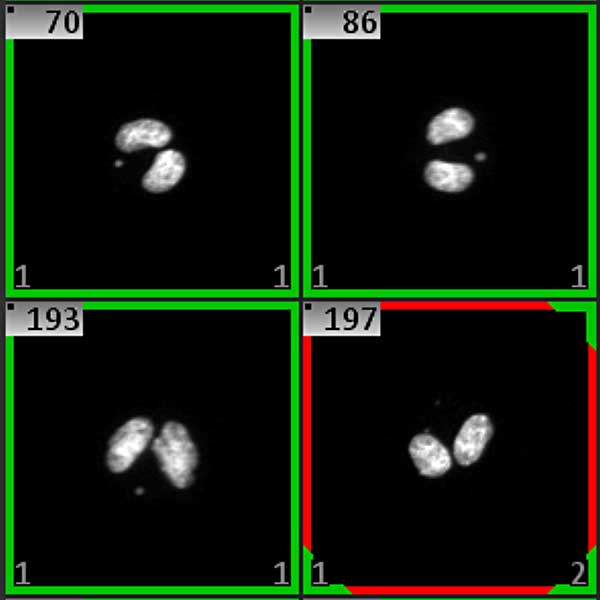
The cytokinesis-block micronucleus assay is a comprehensive method for measuring DNA damage, cytostasis and cytotoxicity. The assay consists in adding cytochalasin-B (an inhibitor of the mitotic spindle that prevents cytokinesis) to cell cultures. As a consequence, cells that have completed one nuclear division are identified by their bi-nucleated appearance. DNA damage events are scored specifically in once-divided bi-nucleated cells.
A scanning system operated by the Metafer software automatically scans in-vitro MN samples, reliably identifies bi-nucleated cells (cells that underwent one cell separation after treatment), and analyzes them for the presence of micronuclei. Simultaneously all mono-nucleated nuclei are counted, providing feedback on the proliferation rate of the culture.
Analysis results are displayed in a cell gallery, showing each single analyzed cell together with the respective micronucleus count. All data are summarized as histogram. Users can select the classes of micronucleus positive cells, filter the gallery by selection and, thus, quickly confirm automated results without the necessity to scroll through all cells. Cells and micronuclei morphologies are analyzed based on user-adaptable parameter sets. Parameter optimization and, thus, setting the scoring standards is done with the help of an automated self-optimization tool. Auto-optimization can also be used for finding the best image processing parameters.
Micronucleus scoring with the Metafer MNScoreX software is not just highly robust, but also extremely fast. It has become a very important tool for radiation biodosimetry. Several independent studies in peer-reviewed journals demonstrate that micronucleus scoring with Metafer MNScoreX is a useful tool for radiation dose estimation, especially in scenarios where a large accident involving sources of ionizing radiation is presumed.
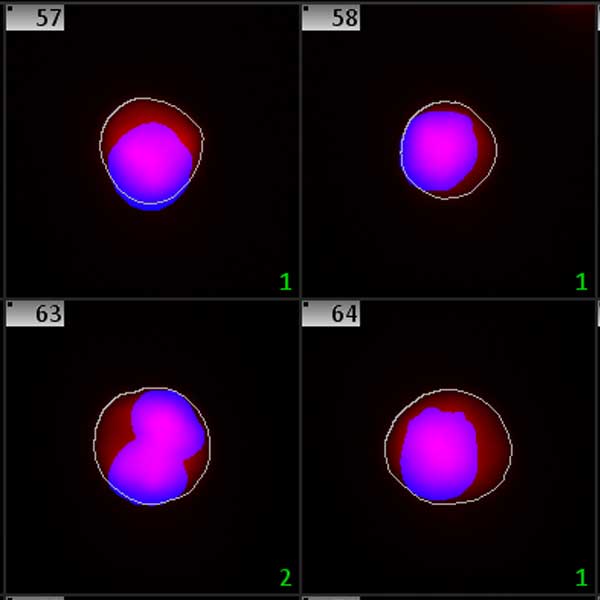
In toxicology, cytostatic effects of the test compound are measured by assessing the ratio of mono-, bi- and multi-nucleated cells (CBPI). This method is also part of the respective OECD guideline (OECD guideline #487). Metafer MNScore automatically analyzes the status of the cells by morphology analysis of the nuclei and calculates the CBPI. The smart workflow is fully compliant with the respective OECD guideline, and also with the GLP regulation framework.
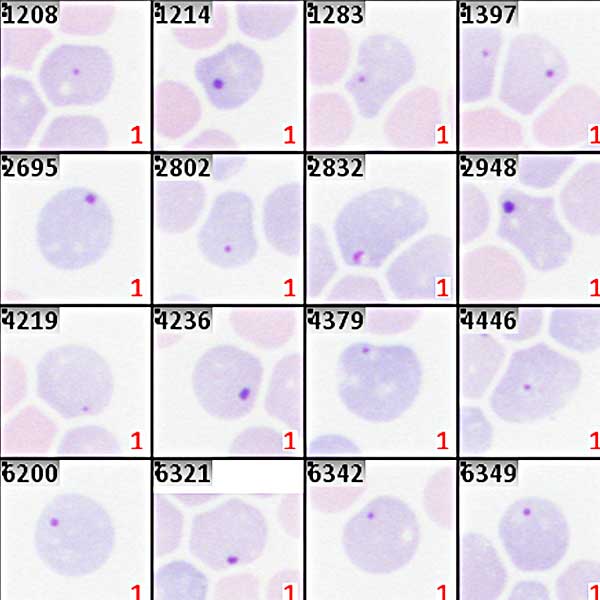
The in-vivo MN test (rodent erythrocyte MN assay) normally uses bone marrow or peripheral blood of rodents which have been treated with the test substance. The red blood cells from the sample are classified as polychromatic (PCE; immature) or normochromatic (NCE; mature) erythrocytes. In PCE the main nuclei have been extruded, and MN remain behind in the otherwise anucleated cytoplasm. An increase in the frequency of micronucleated polychromatic erythrocytes in treated animals is an indication of induced chromosome damage. PCE and NCE are distinguished by color (after applying a May-Gruenwald-Giemsa stain), and the ratio between both populations bears information about cell cycle delays due to toxic side effects of the testing agent. It is important to know that scoring automation of the in-vivo MN test requires samples prepared from column purified cell solutions, and that it is recommended to prepare samples with cyto-centrifugation. Only this way of preparing slides assures reliable and error-free automated analysis.
Automation of the in-vivo MN test with the Metafer software is done in a completely unattended workflow. The scanning system operated by Metafer SCAN automatically acquires images of the sample and analyzes the intensity range of the staining. With reference to the staining quality, PCE and NCE are automatically distinguished based on their color. The scanning workflow is robust enough to cope with varying staining qualities and with different ratios of PCE and NCE (for instance, if peripheral blood is used instead of bone marrow). Once PCE and NCE colors are defined, each cell is assigned to one of the classes, and micronuclei are detected with Metafer MetaCyte. Typically, more than 1,000 cells per minute are scanned and processed.
Automation of the in vivo MN test with Metafer MetaCyte is fully compliant with the respective OECD guideline (OECD guideline #474), and with the GLP regulation framework.
MetaSystems offers Customization Packages for application workflows using standard Metafer platform functionality. The Customization Packages are not intended for diagnostic use.










