in vivo MN Test
The Rodent Erythrocyte Micronucleus Assay

You may benefit most from the following customizations:
- Customizable workflows for the automated analysis of micronuclei for in vivo micronucleus assay preparations.
- Dynamic separation of PCE and NCE populations by color, based on user-defined parameters.
- Independent detection of secondary objects in both PCE and NCE populations, serving as suggestions for calculating the micronucleus rate.
- Calculation of the proliferation rate by determining the ratio of objects classified as PCE or NCE.
- The ability to configure procedures that adhere to the relevant OECD guidelines and are suitable for use in GLP-compliant laboratories.
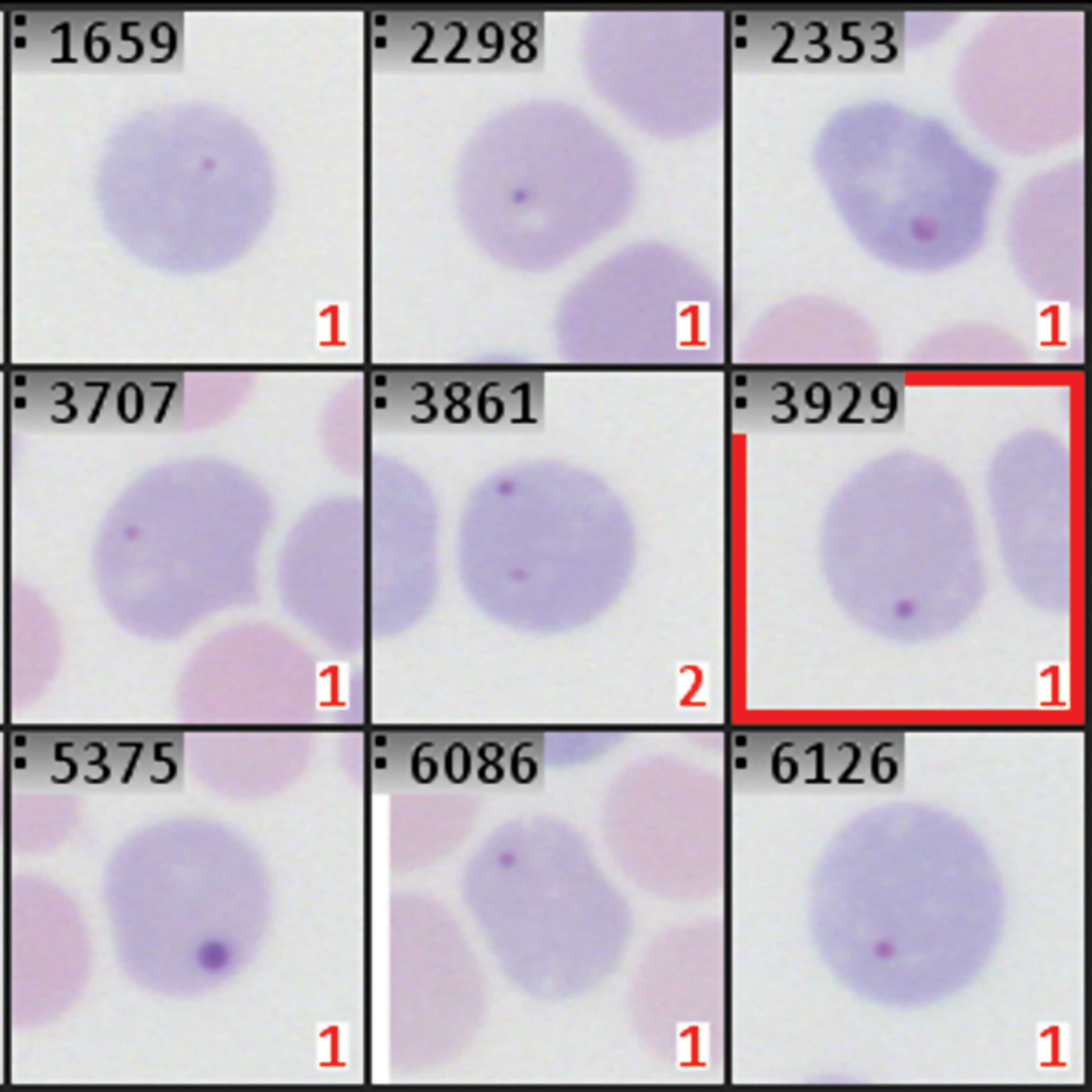
In the in vivo micronucleus test (erythrocyte MN test in rodents), bone marrow or peripheral blood from rodents treated with the test substance is typically used. This test is a key part of preclinical assessments required by regulators for manufacturers of pharmaceuticals, chemicals, and cosmetics in many countries. The test involves classifying red blood cells from the sample as either polychromatic (PCE; immature) or normochromatic (NCE; mature) erythrocytes. In PCEs, the main nuclei are displaced, and micronuclei remain in the otherwise nucleus-free cytoplasm. An increase in micronucleated polychromatic erythrocytes in treated animals indicates induced chromosomal damage.
With the support of our application specialists, many MetaSystems customers have successfully integrated the Metafer Platform Software into their workflows for the in vivo MN test. Automation of this test can be achieved through an unattended digitization process, where Metafer tools analyze the staining intensity range, aiming to separate the PCE and NCE populations based on the results. The scanning workflow can be dynamically adjusted to handle varying staining qualities and different PCE-to-NCE ratios (e.g. when using peripheral blood instead of bone marrow).
Once the PCE and NCE colors are defined, Metafer can be configured to count secondary objects within the respective cell populations as micronuclei. This data is then used to calculate key test parameters, including the micronucleus rate in PCEs, the PCE/NCE ratio, and the total number of objects in each subpopulation. As the digitization process can be fully automated, this workflow enables the analysis of larger cell numbers compared to manual microscopy, resulting in more robust statistical evaluations. As is typical with Metafer-based routines, the results are presented in a comprehensive and filterable object gallery along with summary graphics, allowing a toxicologist to efficiently review the findings on screen.
Many of our users have implemented and validated this workflow, or a similar one, in their GLP-certified laboratories, thereby ensuring that their processes comply with the required regulatory standards. By adopting this approach, they have not only streamlined their workflows but also adhered to the relevant OECD guideline (OECD Guideline #474). This compliance has reinforced the integrity and accuracy of their testing procedures, making their results suitable for submission in regulatory approval processes and industry-specific applications.
Unfortunately not. Manually prepared smear slides have limited potential for standardization, which is a critical requirement for this test. Additionally, unpurified samples often contain unnecessary material, such as nucleated cells, that is irrelevant and even disturbing for the analysis of the in vivo MN test. The best preparations are achieved by combining cellulose column purification with the use of a cytocentrifuge. This method ensures that the preparation contains only erythrocytes, which are laid flat on the slide, preventing artifacts caused by the folded structure of manually dropped or smeared cells.
Our customers have encountered that the initial additional cost and time needed to produce such preparations are quickly offset by the significant time savings achieved through the automation of much of the test process with Metafer-based workflows. The use of a cytocentrifuge offers the added benefit of concentrating the material in a clearly defined area of the preparation, which can be incorporated into the Metafer Platform Software. This approach can further reduce scanning times considerably.
The May-Gruenwald stain is a differential stain used for air-dried blood and cytological preparations. The May-Gruenwald solution is a mixture of methylene blue, eosin, and methanol, dissolved in water. When combined with the Giemsa stain, it forms the May-Gruenwald-Giemsa stain. This stain causes the cytoplasm of mature erythrocytes to appear bright red, while the cytoplasm of immature erythrocytes takes on a slight bluish tint. As a result, May-Gruenwald staining provides a useful method for differentiating between these two subpopulations.
Unfortunately, it is common for preparations stained with May-Gruenwald-Giemsa to vary in intensity and color. This variation is critical because the color values of erythrocytes are used to differentiate between the PCE and NCE populations. In our proposed workflow, this issue is addressed by having Metafer first analyze the staining quality of a user-defined number of erythrocytes on each slide. This data is then used to define the color range within which each population falls. Only after evaluating the defined number of cells are all subsequent cells assigned to one of the two populations. Since the boundary between the two populations is gradual, cells that fall between the populations are excluded from the analysis, ensuring that only those cells that can be clearly identified as belonging to one of the two populations are recorded.
MetaSystems software provides, among other functions, features to assist users with image processing. These include, but are not limited to, the use of machine and deep learning algorithms for pattern recognition. The output generated in this process should be regarded as preliminary suggestions and, in any case, mandatorily requires review and assessment by trained experts.
MetaSystems offers Customization Packages for application workflows that have been successfully implemented for customer labs using standard Metafer platform functionality. It is expected that they can be implemented for other customer labs using similar workflows and slide preparation procedures. If a Customization Package is purchased, MetaSystems product specialists will – based on their experience from other similar application cases - support the customer lab in adapting the Metafer software configuration to their needs. The performance of the solution will depend on the quality of the customer slides and the expertise of the users, MetaSystems cannot specify or guarantee any performance parameters. The validation of the solution for clinical use is the sole responsibility of the customer lab.