Ames Test
Ames II / Ames MPF
You may benefit most from the following customizations:
- Unattended scanning of Ames II / Ames MPF Microwell Plates.
- Recording of color values of the individual wells for the detection of bacterial colonies.
- Customizable reports of the results in the form specified by the respective test manufacturer.
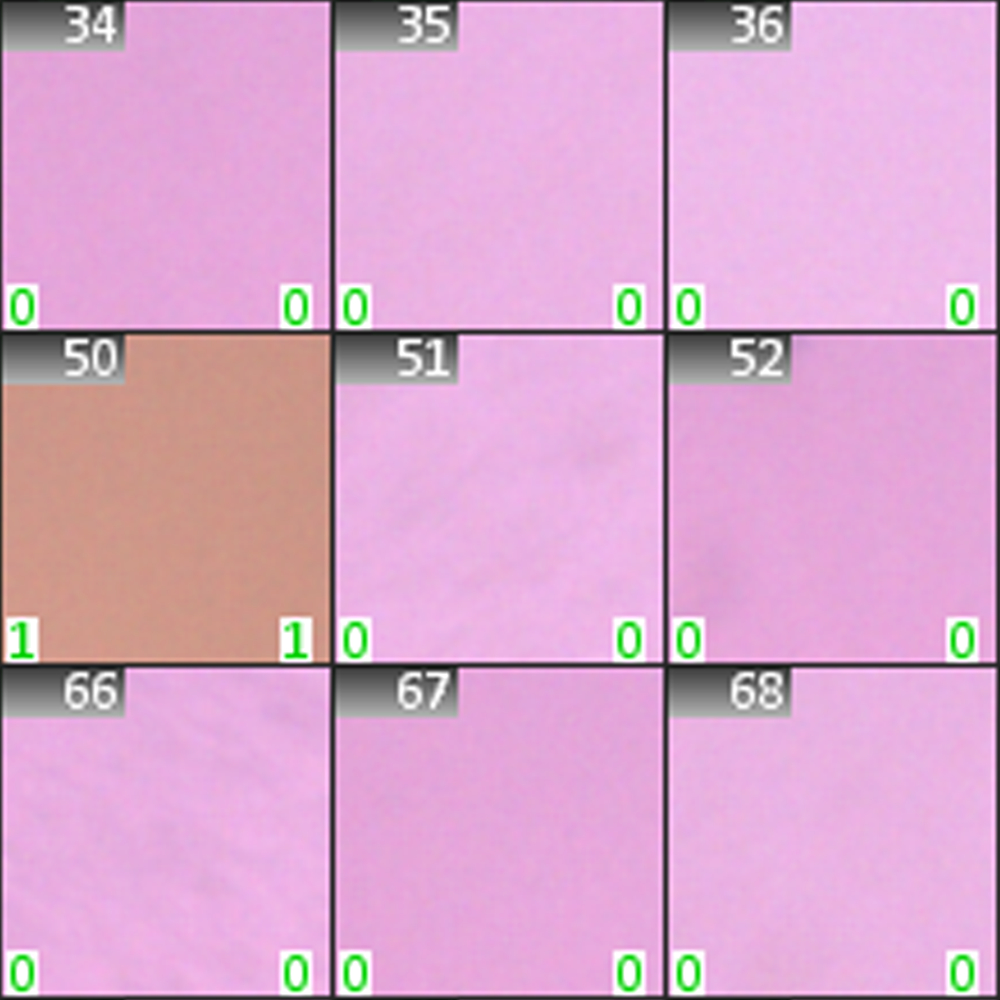
The Ames test is a biological assay used to evaluate the mutagenic potential of chemical substances. It involves exposing mutant strains of bacteria, which cannot produce a specific amino acid (e.g., histidine), to the test substance. These bacteria are cultured on a medium lacking the amino acid, preventing normal growth. If the substance causes genetic mutations that restore the bacteria’s ability to produce the amino acid, colonies will form, indicating a reverse mutation. In a modern variation of the Ames test, bacteria are grown in indicator media on microwell plates, where changes in media color signal bacterial growth.
Our users leverage the imaging capabilities of the Metafer Platform Software to scan these microwell plates and analyze the color changes in individual wells. The results of such scans help identify and quantify positive cultures, providing valuable data on Ames test outcomes. Metafer can be tailored to generate detailed reports from this data, formatted according to the specifications of the test kit manufacturer. These reports, along with raw data and results from other studies on the same test substance, can be archived to create a comprehensive view of the toxicology study.
Yes, in a traditional manual evaluation of the Ames test, bacteria are spread on Petri dishes, and colonies are counted under a microscope. However, modern versions of the test, optimized for high throughput, use microwell plates where each well represents a culture. By scoring the wells, the number of colonies can be determined much faster. Metafer users benefit from the software's ability to automate this process by scanning the wells, analyzing the color values of the indicator medium, and providing condition assessments for each well. Since this workflow is entirely digital, it offers not only faster processing but also comprehensive documentation of results, which can be easily compiled into detailed reports.
MetaSystems software provides, among other functions, features to assist users with image processing. These include, but are not limited to, the use of machine and deep learning algorithms for pattern recognition. The output generated in this process should be regarded as preliminary suggestions and, in any case, mandatorily requires review and assessment by trained experts.
MetaSystems offers Customization Packages for application workflows that have been successfully implemented for customer labs using standard Metafer platform functionality. It is expected that they can be implemented for other customer labs using similar workflows and slide preparation procedures. If a Customization Package is purchased, MetaSystems product specialists will – based on their experience from other similar application cases - support the customer lab in adapting the Metafer software configuration to their needs. The performance of the solution will depend on the quality of the customer slides and the expertise of the users, MetaSystems cannot specify or guarantee any performance parameters. The validation of the solution for clinical use is the sole responsibility of the customer lab.