Comet Assay / Foci
Single Cell Based DNA Damage Analysis
Automation of quantitative DNA lesion analysis based on single cells.
Toxicologists and radiation biologists nowadays can choose from many established tests to assess DNA damage. However, many of these tests are based on counting events that are only indirectly related to the original lesion. Chromosomal aberrations and micronuclei, for instance, are caused by DNA breaks, but the microscopically visible structure is the outcome of complex cellular processing. Comet assay and γ-H2AX foci analysis, however, bear the chance to directly assess the damage on a single cell level. As part of the toxicology portfolio, MetaSystems automated both assays in smart scanning workflows managed by the Metafer software.
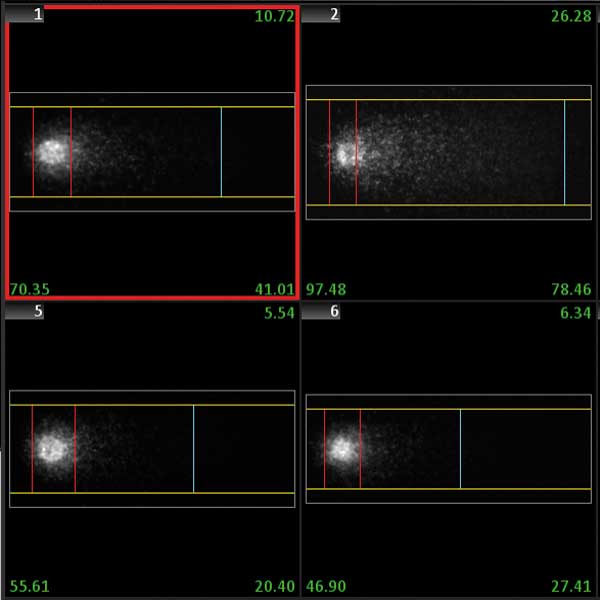
The Comet (single cell gel electrophoresis) assay is a method for obtaining the amount of DNA fragmentation of single cells. It is widely used to estimate DNA damage caused by chemicals or radiation, for example to test individual sensitivities to ionizing radiation in cancer patients. The assay involves image analysis of the migration pattern caused by fragmented DNA in an agarose gel. The more fragmented the DNA is, the more the DNA fragments migrate, forming a structure that resembles the tail of a comet. Damage quantification is then based on the calculation of the 'head' (the actual cell) and the 'tail' (the migrated fragments).
The smart scanning workflow with the Metafer CometScan package automatically detects and selects cells in single cell gel electrophoresis samples. All selected cells are analyzed for all features important for the Comet assay (tail moment, Olive tail moment, %DNA in tail, tail and head dimensions, and many more). The background conditions in the images are measured, and results data can be corrected for uneven background. Comets are detected based on user-defined morphological criteria and thresholds between cells and background. Detected comets are displayed in the cell gallery, and each comet can be relocated with a single mouse click. Data are displayed in convenient histograms and/or scatter plots, which are continuously updated as the scan progresses. The smart scanning workflow supports multiple exposure times for image acquisition. Images having an extended dynamic range for more precise and reliable measurements.

Metafer CometScan is the perfect software to support the automated, high-throughput Comet assay analysis. It supports a variety of slide with different dimensions, e.g., to image multi-gel specimens.
If the signal analysis features within Metafer MetaCyte are used, there are many more options for automated target cell selection. For example, such a scanning workflow can automatically detect Hedgehog Comets and distinguish their results from the other cells.
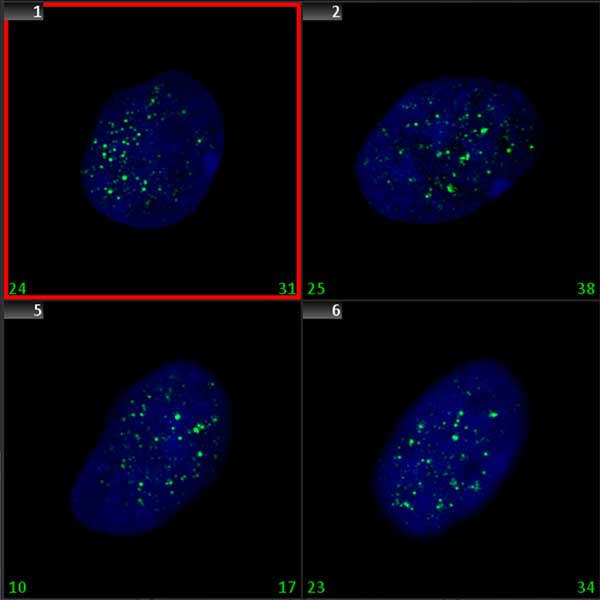
Double-strand breaks (DSB) are the most deleterious DNA lesions that, if left unrepaired, may have severe consequences for cell survival. They are known to lead to chromosome aberrations, genomic instability, and cell death. Various physical, chemical, and biological factors are involved in DSB induction. The histone H2AX, a protein contributing to the nucleosome-formation and therefore the structure of DNA, becomes phosphorylated on serine 139, then called γ-H2AX, as a reaction to the formation of DSBs. Since γ -H2AX expands a large distance from the DSB site, and since specific γ-H2AX antibodies are available, the DSBs can be visualized as discrete foci. Quantification of γ-H2AX foci can be used to detect the genotoxic effect of different toxic substances, including ionizing radiation.
Since γ -H2AX foci microscopically appear like spot shaped signals, they can be automatically analyzed using the signal counting functionality in Metafer MetaCyte. In contrast to FISH spots, however, γ-H2AX foci tend to vary in size, and also to fuse into larger patches of fluorescent staining in cells exposed to higher doses of a DSB inducing agent. The Metafer MetaCyte module counts foci in cell nuclei directly and automatically, but it also offers the possibility of adding a weighing term into the measurement. Thus, it allows for adaptation of scoring conditions to the own scoring standards. Adaptation can be done using data from validation experiments with valid dose-effect curves.
MetaSystems offers Customization Packages for application workflows using standard Metafer platform functionality. The Customization Packages are not intended for diagnostic use.










